
Using XEOMIN Step‑by‑Step
XEOMIN Offers Convenience for Your Practice
Offered in 3 different vial options:
50, 100, 200 Units1
Packaged to help
minimize waste
Does not require refrigeration
prior to reconstitution1
Reconstitution and Dilution—Flip It. Don't Shake It.
- Prior to injection, reconstitute each vial of XEOMIN with sterile, preservative-free 0.9% Sodium Chloride Injection, USP1
- A 20- to 27-gauge short-bevel needle is recommended for reconstitution
- Draw up an appropriate amount of preservative-free 0.9% Sodium Chloride Injection, USP into a syringe1

Step 1: Vial preparation
Clean the exposed portion of the rubber stopper of the vial with alcohol (70%) prior to insertion of the needle.1

Step 2: Saline injection
After vertical insertion of the needle through the rubber stopper, the vacuum will draw the saline into the vial. Gently inject any remaining saline into the vial to avoid foam formation. If the vacuum does not pull the saline into the vial, then XEOMIN must be discarded.1

Step 3: Mixing
Remove the syringe from the vial and mix XEOMIN with the saline by carefully swirling and inverting/flipping the vial—do not shake vigorously.1

XEOMIN Reconstitution Video
Reconstituted XEOMIN is a clear, colorless solution free of particulate matter. XEOMIN should not be used if the reconstituted solution has a cloudy appearance or contains floccular or particulate matter.1
Storage—No Refrigeration Required1
- Refrigeration of unopened vials is not required
- Unopened vials of XEOMIN should be stored at or below 25°C (77°F)
- Do not use after the expiration date on the vial
- Reconstituted XEOMIN may be stored in a refrigerator at 2° to 8°C (36° to 46°F) for up to 24 hours until time of use
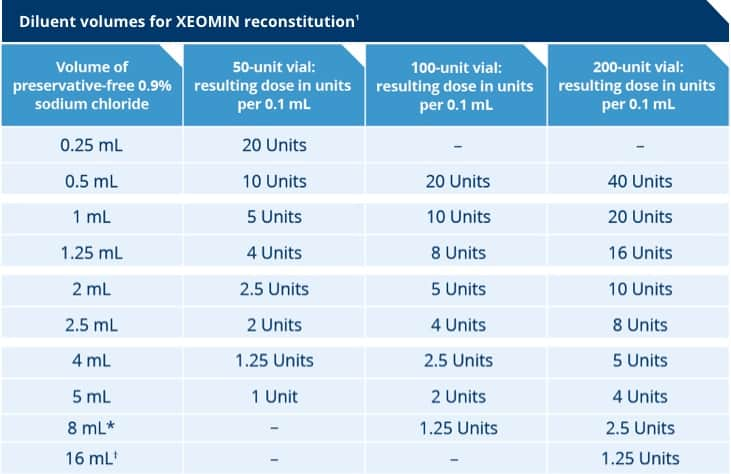

*When using 8 mL of diluent for a 100 Unit or 200 Unit vial of XEOMIN, complete the following steps:
- Reconstitute a 100 Unit or 200 Unit vial of XEOMIN with 4 mL of preservative-free 0.9% Sodium Chloride Injection, USP, following instructions above.
- Withdraw 4 mL of preservative-free 0.9% Sodium Chloride Injection, USP, into an appropriately sized syringe for 8 mL in total.
- Using the same syringe, draw up the 4 mL of XEOMIN solution from the reconstituted vial and mix gently.
†When using 16 mL of diluent for a 200 Unit vial of XEOMIN, complete the following steps:
- Reconstitute a 200 Unit vial of XEOMIN with 4 mL of preservative-free 0.9% Sodium Chloride Injection, USP, following instructions above.
- Withdraw 12 mL of preservative-free 0.9% Sodium Chloride Injection, USP, into an appropriately sized syringe for 16 mL in total.
- Using the same syringe, draw up the 4 mL of XEOMIN solution from the reconstituted vial and mix gently.
Administration Instructions1
- Intended for intramuscular or intra-salivary gland injection only
- Should be used for only 1 injection session and only 1 patient
- For intramuscular injections, the number of injection sites is dependent upon the size of muscle to be treated and the volume of reconstituted XEOMIN injected. A suitable sterile needle (eg, 27‑30 gauge [0.30‑0.40 mm diameter], 12.5 mm length) should be used for intra-salivary gland administration for the treatment of chronic sialorrhea
- The salivary glands can be located using ultrasound imaging or surface anatomical landmarks
- Reconstituted XEOMIN solution should be administered within 24 hours after dilution. During this time period, reconstituted XEOMIN should be stored in a refrigerator at 2° to 8°C (36° to 46°F)
Important: Do not pry or pop the cap off of the XEOMIN vial as it is vacuum packed and doing so will result in XEOMIN powder dispersing into the air.
Reference
- XEOMIN® [Package insert]. Raleigh, NC: Merz Pharmaceuticals, LLC; 2021.
